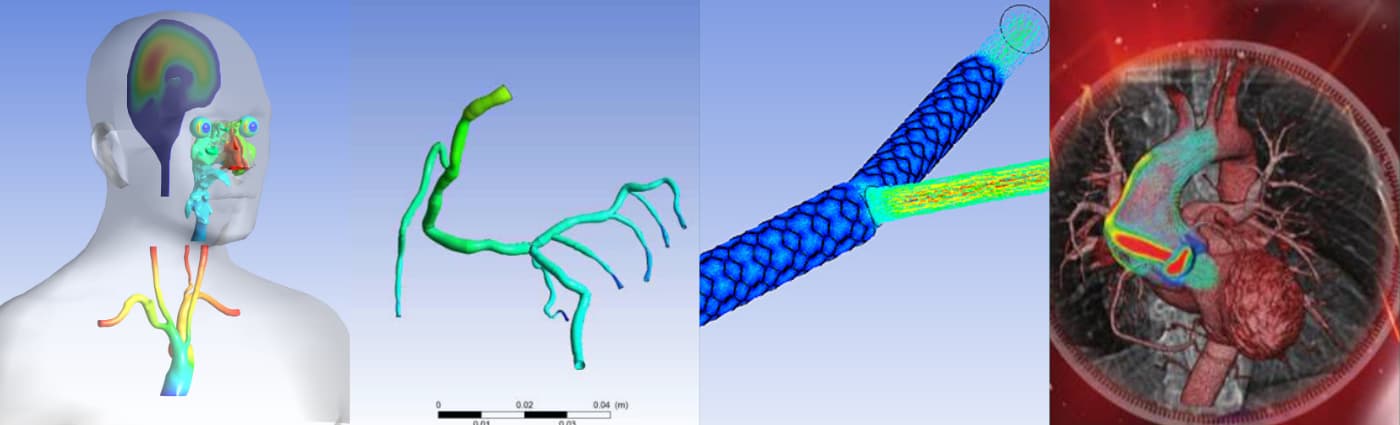
Bringing a new healthcare product to market is a race against time in a very regulated environment. Innovation is key. But so is speed. Unique ideas, painstaking research, effective trials and even safe, fully approved products will mean little if someone else gets there first. For example, there are no shortcuts for the research it takes to develop the precise medical devices or treatment required for today’s market — but there is a way to shorten the time it takes to perform that research. Simulation testing developed by ANSYS can assist and reduce more costly in vitro and in vivo testing. It allows professionals in the healthcare industry to use virtual prototyping to drive product development. At the clinical trial phase, modeling advanced interactions means tests can be run efficiently and effectively, without compromise, throughout the complex government approval process. Thanks to continuous progress, the latest applications are used in diagnosis and patient-specific medicine, which combine engineering simulation with medical imaging and other sophisticated data. ANSYS has a proven track record of accurately modeling physics interactions and performing automatic design exploration analysis. By optimizing the opportunity for success throughout the research and development phase, innovative products and processes can get to market more quickly while complying with strict medical regulations, ultimately saving money and time — two components critical for success in today’s fast-moving markets.
Please specify "Source Advantech, Jsc." or "According to www.advantech.vn" if you want to disseminate this information


